We have outlined points for consideration when developing an immunogenicity assay in Table 1 For full descriptions of the technical advantages and disadvantages of the one or two assay approach for PK and ADA, please refer to Marini etA typical suite of assays required during biotherapeutic development includes drug concentration assay (pharmacokinetic or PK assay) and assays detecting presence of antidrug immune response Multiple assays may be required to determine appropriate compound PK as well as to characterize an antidrug immune response As such, PK assays are a key component of the drug development process with the global PK assay service market currently valued at US $6528 million (link is external)PK assay results are critical in all phases of drug development, starting from target/molecule selection during the discovery stage, through PK projections from animal to human to inform doses for early

Pk And Ada Assay Development Services
Pk assay development and validation
Pk assay development and validation-To support PK studies at all phases of development, we offer assay development, validation, and implementation using ELISAs and MesoScale Discovery (MSD) assays for biologics, and flow cytometry and Droplet Digital PCR (ddPCR) for cellbased therapies33 PK/PD relationship development and use the same assay(s) during the entire development program The difficulty of developing such an assay at an early stage is, however, recognised Methods should be adequately validated prestudy and withinstudy according to standard practice1,2 Difficulties may arise in the bio



1
Here we describe the development of pharmacodynamic (PD) assays to quantify MASP2 inhibition ex vivo and their use to study duration of action and to establish the pharmacokinetic (PK)/PD relationship of narsoplimab in monkeys and humans Methods PD assays to measure lectin pathway activity, a measure of MASP2 activity, in minimally diluted Currently, those recommendations are widely adapted for PK LBAs However, for development and qualification of LBAs measuring endogenous proteins such as biomarkers and 'free'/'total' drug targets, the method development and qualification strategies will differ from the approach accepted for PK assaysMESO SCALE DISCOVERY® offers a range of assay development materials and kits suitable for the implementation of immunogenicity assays, neutralization assays (binding, cellbased, and cytokine assays) and PK assays The MSD® platform, based on electrochemiluminescence, provides excellent sensitivity, a large dynamic range, and flexibility
Biomarker Testing Services using Commercial Assays and Kits NorthEast BioLab provides biomarker testing services with an exhaustive menu of 600 analytes in the usual biological matrices, given the attractive risk/reward profile of commercial assays We offer biomarker analysis services using a singleplex or multiplex panel for a wide range ofOur scientists have longstanding experience in PK biomarker assessment including assay development and validation under GLP quality standards The result of PK biomarker assessment studies at Creative Biolabs is accurate and reliable to ensure highquality PK assay services for our clients all over the worldPharmacokinetics (from Ancient Greek pharmakon "drug" and kinetikos "moving, putting in motion";
PK/PD assays Active Biomarkers Understanding, on the one hand, what the body does to the therapy (ie, Pharmacokinetic, PK), and, on the other hand, what the therapy does to the body (ie, Pharmacodynamic, PD) is essential to maximize the chances of successful development of your drug Linking therapy exposure to the pharmacodynamic effectThe tutorial introduces the readers to the fundamentals of antibody pharmacokinetics (PK) in the context of drug development Topics covered include an overview of antibody development, PK characteristics, and the application of antibody PK/pharmacodynamics (PD) in research and development decisionmakingCharacterization assay (class/isotypes of antibodies, neutralizing yes/no) If the pharmacokinetics (PK) of the active ingredients are also available, possible changes in the PK may be correlated with the effects induced by ADA vivo Science offers the complete development, implementation, and optimization of ADA assays
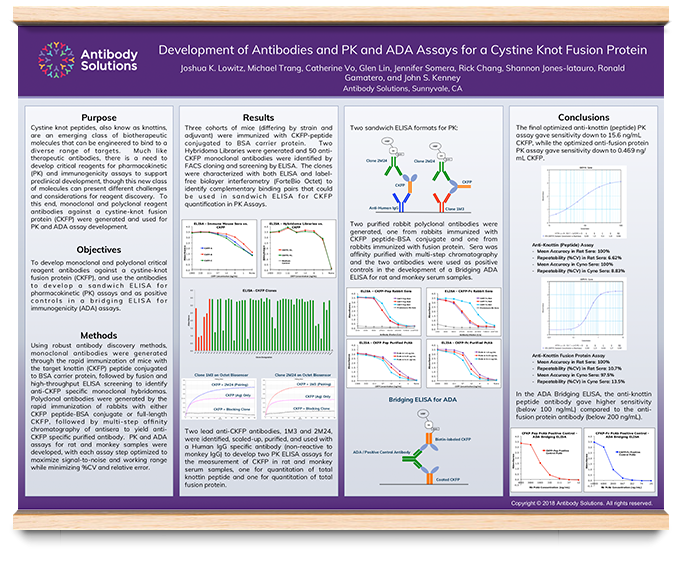



Development Of Antibody And Pk And Ada Assays For A Cystine Knot Fusion Protein




Pk Clinical Trial During New Drug Discovery And Development An Over
Gyrolab Assay Protocols Gyrolab systems are supported by an increasing range of readytouse kits, and yet remain as open systems for enabling the inhouse development and evaluation of highperformance custom immunoassaysTo help you accelerate development of commonly used immunoassays, readytouse and robust Gyrolab assay protocols are now available for usePharmacokinetics (PK) studies are of great importance in creating new therapeutic drugs, understanding both the beneficial and harmful effects of drugs, and guiding drug development experiments and trials In PK assays, the applied assay method should be well characterized and fully validated, in order to yield reliable resultsBioanalytical Method Validation 05/24/18 Bioanalytical Method Validation Guidance for Industry US Department of Health and Human Services Food and Drug Administration




Pharmacokinetic Studies In Children Recommendations For Practice And Research Archives Of Disease In Childhood



Establish Drug Pk Analysis Method Based On Competitive Elisa
Assay Development Overview Analytes References Quick Links New Products;Find MilliporeGFDX MSDS, related peerreviewed papers, technical documents, similar products & more at SigmaAldrichSee chemical kinetics), sometimes abbreviated as PK, is a branch of pharmacology dedicated to determine the fate of substances administered to a living organism The substances of interest include any chemical xenobiotic such as pharmaceutical drugs, pesticides, food additives,



Pk Pd Modeling Of Protein Drugs Implications In Assay Development Bioanalysis



Frontiers Computational Approaches In Preclinical Studies On Drug Discovery And Development Chemistry
Method development of a novel PK assay for antibodyconjugated drug measurement of ADCs using peptidelinker drug analyte SukJoon Hyung Department of BioAnalytical Sciences Assay Development and Technologies, Genentech, Inc, South San Francisco, CA, , USAPK PD Analysis Services PK PD analysis study examines a drug's effect compared to its exposure in the body after dosing PK PD assays estimate the safety and efficacy of therapeutics after suitable bioanalysis Pharmacokinetics modeling and simulation help further understand what the body does to a drug, modeling the processes of absorptionThe most optimal approach is to develop a single PK assay, using a single analytical standard, for quantitative measurement of the biosimilar and reference products in serum matrix Use of a single PK assay for quantification of multiple products requires a scientifically sound testing strategy to evaluate bioanalytical comparability of the




Pk Assay Analysis Services Pharmacokinetics Pk Study Pk Testing Northeast Biolab



Method Development Of A Novel Pk Assay For Antibody Conjugated Drug Measurement Of Adcs Using Peptide Linker Drug Analyte Springerlink
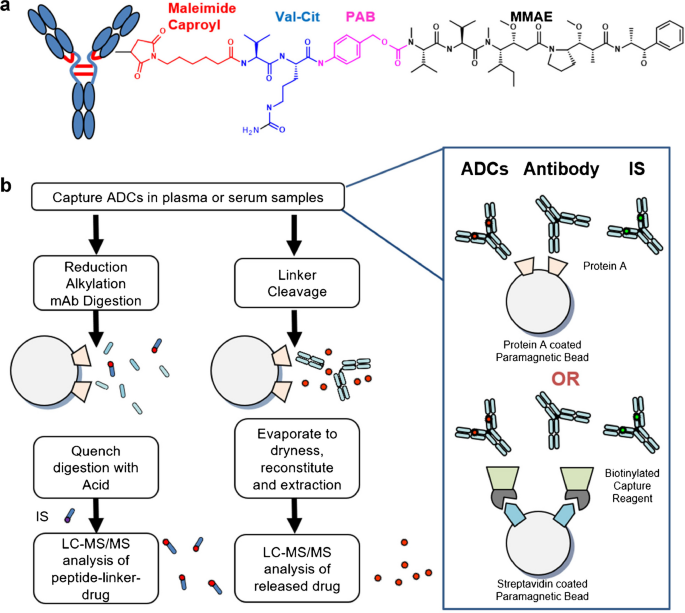
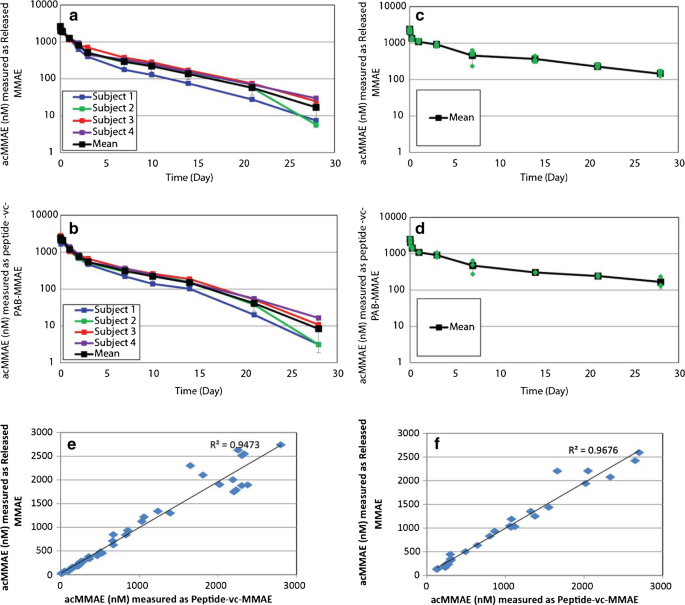
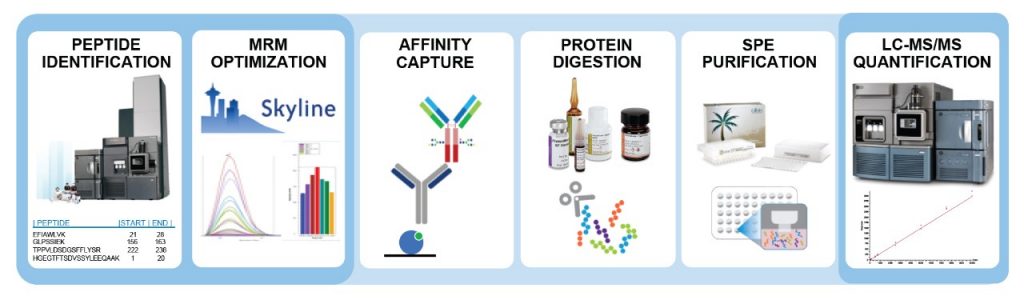
Development of acDrug assay using peptidelinker drug as surrogate analyte To expand the use of the acDrug bioanalytical strategy to ADCs with noncleavable linkers, we first sought to develop a method that measures concentration of acDrug using peptidelinker drug as the analyte where it can directly be compared to the released drug Commercial assay development kits are available to ease the assay establishment For example, Stadtmauer EA et al utilized the DuoSet Ancillary Reagent Kit from R&D Systems to establish an ELISA assay detecting residual Cas9 protein in CRISPRengineered T cells Commercial organizations have developed a wide array of assays, and should firstJohn L Allinson, FIBMS, believes despite the increased use of biomarkers, it appears that many researchers are still continuing to use the FDA guidance document for validation even though it only critically addresses the validation of assays to support PK evaluation, and also has a limited scope described within the document in terms of studies where it should be used




Pk Pd Modeling Of Protein Drugs Implications In Assay Development Bioanalysis




Improving The Accuracy Of Predicted Human Pharmacokinetics Lessons Learned From The Astrazeneca Drug Pipeline Over Two Decades Trends In Pharmacological Sciences
PK assay bioanalytical testing methods are used to determine concentration time profiles of the drug and metabolites in biological sample fluids, providing information necessary for PK analysis PK assays are a vital component of the drug development process, and the data derived is used to help select dosage for preclinical and clinical studiesDevelopment of Antibody and PK and ADA Assays for a Cystine Knot Fusion Protein Joshua K Lowitz, Michael Trang, Catherine Vo, Glen Lin, Jennifer Somera, Rick Chang, Shannon JonesIatauro, Ronald Gamatero, and John S Kenney Antibody Solutions, Sunnyvale, CA, USAFor more information, visit http//wwwbioradcom/HuCALAntiDrugAntibodiesDr Achim Knappik, R&D Group Manager at BioRad Laboratories, describes the use



Immunogenicity And Pk Pd Evaluation In Biotherapeutic Drug Development Scientific Considerations For Bioanalytical Methods And Data Analysis Bioanalysis
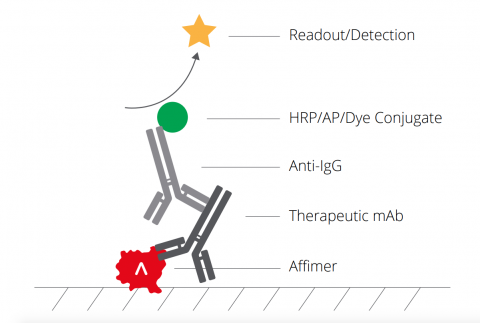



Improving Drug Development Processes With Antibody Pk Assays Avacta Life Sciences Limited
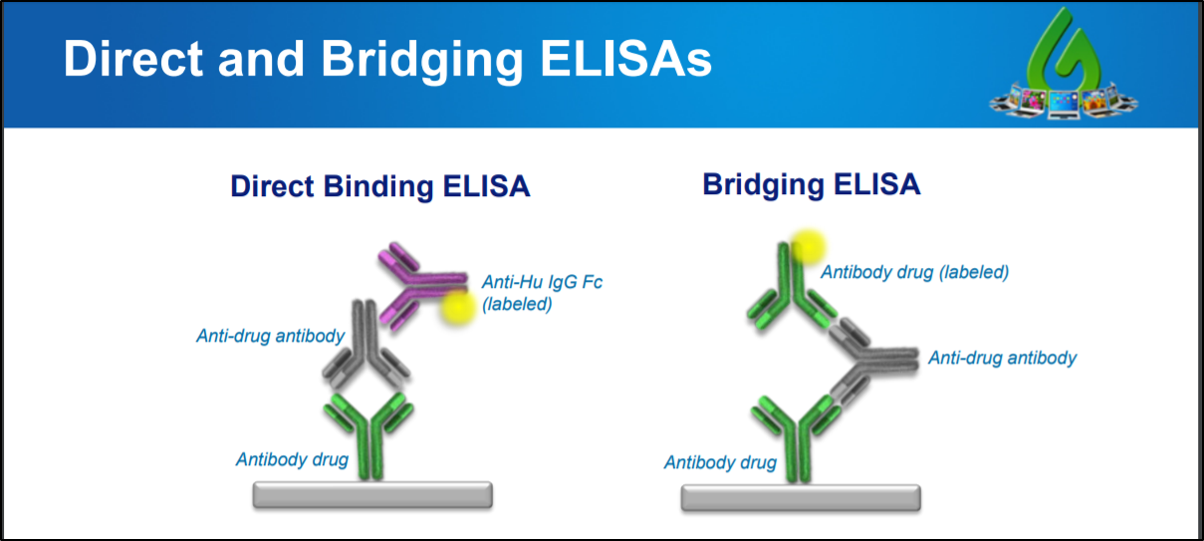
BioRad's antibiotherapeutic antibodies are ideal for the measurement of monoclonal antibody drugs and biosimilar products in PK and ADA assays We have developed a range of highly specific, recombinant antiidiotypic antibodies with different binding modes and properties, enabling the development of bridging ELISA and antigen capture ELISAPharmacokinetics and Immunogenicity ELISA Kit Development Service GenScript ProBio guarantees to deliver highquality PK and ADA assay for measurement of free drugs, binding drugs, total drugs and antidrug antibody in both discovery and clinical stages We offer a complete PK kit for the of the drug discovery and development processDevelopment plan and product labeling Study of the Pharmacokinetics (PK) and Pharmacodynamics (PD) of the drug in humans Assay Validation & Performance Reports



2



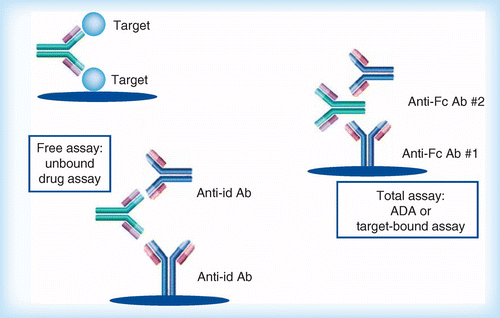
What Is An Anti Idiotypic Antibody
The Europium channel was 06% The AlphaPlex Development Guide provides indepth information for advanced users to further develop their own assays Results Factors to Consider When Setting Up PK Assays with Alpha When using the same antibody on both the donor and acceptor beads, occasionally, the Hook effect is amplified resulting in aThis is a technical position in Pharmacokinetics (PK) Assay Development within the Bioanalytical Sciences group Developing, validating, and conducting bioanalytical methods related to large molecule biotherapeutic drug development, spanning nonclinical studies to postmarket clinical developmentPK/PD/ADA Antibodies and Assays Because all biological therapeutics can induce an immune response that ranges from benign to severe adverse effects, evaluating unwanted immunogenicity is a critical step in earlystage researchrequiring both a wellconceived strategy and fitforpurpose assays for antibody detection and characterization
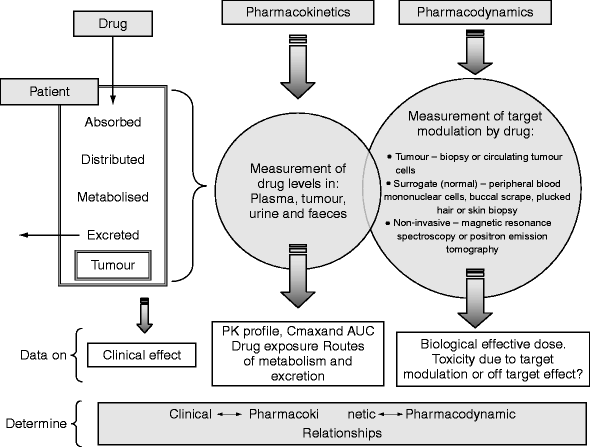



Pharmacokinetics And Pharmacodynamics In Drug Development Springerlink




Pharmacokinetic Assay An Overview Sciencedirect Topics
Development of PK assays for quantitative measurement of the biosimilar and reference products in serum matrix is a critical component to the in vivo characterization of the test products The bioanalytical data are a key element to demonstrating simi Trending QC Performance for PK Assays For PK assays, typically three levels of QC (HQC, MQC, and LQC) which span the quantitative range of the assay are included for monitoring run performances (1,24) The accuracy of QC data is assessed using %RE with acceptance limits of ± % An example of PK assay QC performance trending is presented inThe PK Assay group Develops and validates assays to measure biopharmaceutical products in biological matrices from nonclinical and clinical studies The primary responsibility for this position will be to develop immunological methods for supporting nonclinical and clinical PK



1




Figure 2 From Rapid Development Of Multiple Fit For Purpose Assays On An Automatic Microfluidic System Using A Streamlined Process In Support Of Early Biotherapeutics Discovery Programs Semantic Scholar
PK sample analysis SYRINX Bioanalytics has extensive experience of developing new methods using stateoftheart immunological and cell based techniques for pharmacokinetic (PK) sample analysis SYRINX offers a multistep approach for PK assay development and routine analysis After each step a report is sent to the sponsor for evaluationPharmacokineticpharmacodynamic (PKPD) modeling is an integral part of the preclinical and clinical development of protein drugs Bioanalytical data from appropriately selected and wellcharacterized PK and PD biomarker assays can be incorporated into mechanistic PKPD models and allow a quantitative relationship between protein drug exposure, target modulation, andEnvigo were asked by a client to develop and validate a PK/TK assay for a monoclonal antibody used to treat cancer at levels as low as 10 ng/ml in cyno samples The company first transferred the assay from ELISA to the MSD platform before finally transferring it to Gyrolab™ system, where the development and validation work was completed



Resources Perkinelmer Com Lab Solutions Resources Docs App Alphalisa Pharmacokinetic Tnfa Pdf




Pk And Ada Assay Development Services
You may have seen that in January, the FDA released new guidance designed to facilitate the development and validation of assays for the detection of antidrug antibodies (ADAs) supporting immunogenicity testing of therapeutic protein products during clinical trials The risks to patients that have an ADAgenerating immune response can be highly variable, fromGlass Fiber Diagnostic Pad 10mm x 300 mm strips 100 pk Lateral Flow, Assay Development, lateral flow immunoassays, lateral flow assay device, Immunoassay IVDs;Frequently, the ADA or immunogenicity assay development is an afterthought, as the primary focus on a project is to understand the absorption, distribution, metabolism, and excretion (ADME) of a dosed therapeutic Additionally, understanding the pharmacodynamics of the drug allows for investigators to make critical decisions regarding go/nogo




Nonclinical Drug Development Chris H Takimoto Md Ph



1
This position will be in the Pharmacokinetics (PK) Assay Development group within the Biologics Development Sciences The PK Assay groupDevelops and validates assays to measure biopharmaceutical products in biological matrices from nonclinical and clinical studiesThe primary responsibility for thAssay development for preclinical and clinical biotherapeutic PK studies can sometimes be tedious and time consuming, delaying project timelines This webinar will present a case study highlighting a fitforpurpose and fastturnaround reagent pairing experimental design using the Gyrolab ®Application of PK in optimizing drug therapy and evaluating bioavailability was truly made possible by the skills of the analytical chemists who pioneered the development of HPLC in the 1970s During the 1980s, HPLCUV based assays routinely provided the plasma concentration data that were used to define drug exposure in




Table I From Fit For Purpose Method Development And Validation For Successful Biomarker Measurement Semantic Scholar
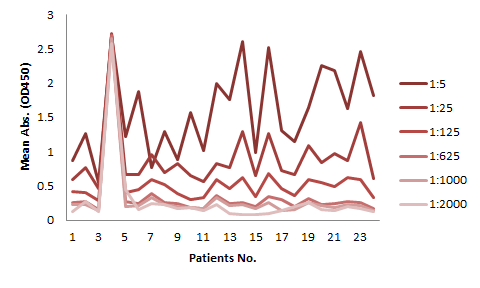



Elisa Assay Kits For Therapeutic Antibodies Acrobiosystems
Pharmacokinetic–pharmacodynamic (PK–PD) modeling is an integral part of the preclinical and clinical development of protein drugs Bioanalytical data from appropriately selected and wellcharacterized PK and PD biomarker assays can be incorporated into mechanistic PK–PD models and allow a quantitative relationship between protein drug exposure, targetBrowse Our Products By Analytes By Applications Search Contact Information Customer Service/Orders 500 am toAll assay developments result in the delivery of a custom kit a protocol, and a fixed quantity of reagents to perform an agreedupon number of tests In as little as 4 to 8 weeks, we will develop and deliver your customized assay
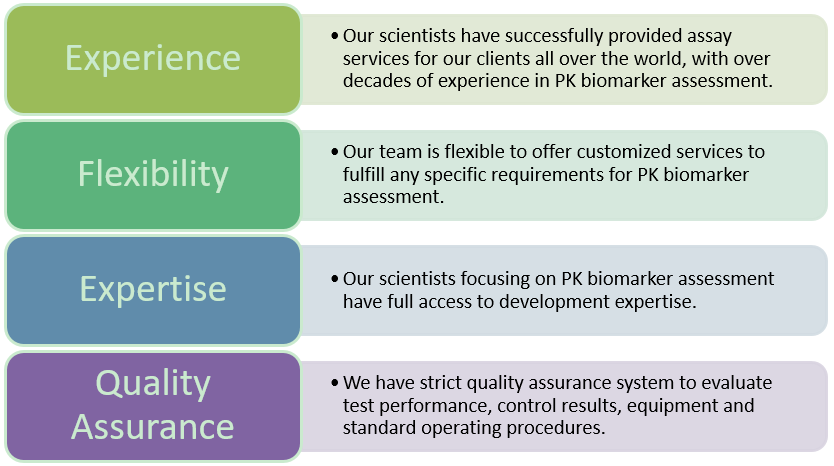



Pk Biomarker Assessment Creative Biolabs
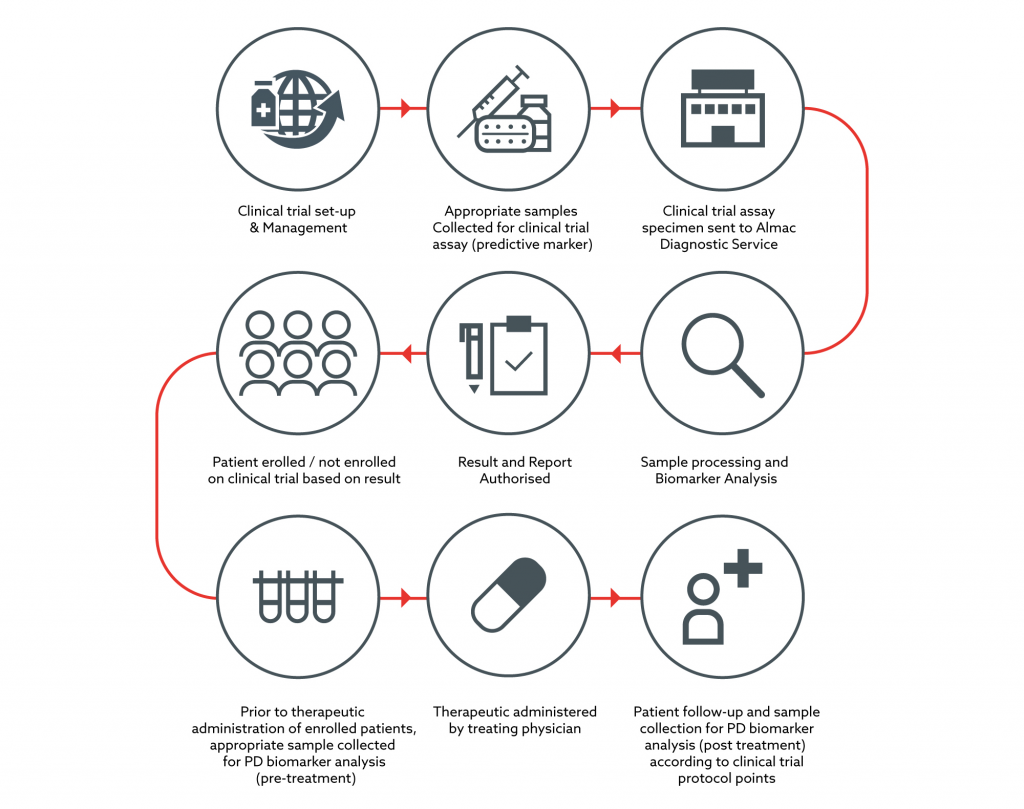



Pharmacodynamic Biomarker Testing Almac
PK/TK Assay Development & Qualification ADA and NAB Assays CMC Support for Biotherapeutics Regulated Bioanalysis and CRO Oversight Quantitative Biomarker and CLIA Assays The B2S Laboratory and Headquarters is located in Franklin, Indiana, USA This laboratory gives you access to a broad range of innovative technology providing you with the



Http Www Genesisbiotechgroup Com Pdf Drug Discovery Services Pdf




Figure 10 From Development Of Pharmacokinetic Pk Assays For Detecting Biosimilars Targeting Tnfa Using Alphalisa Semantic Scholar
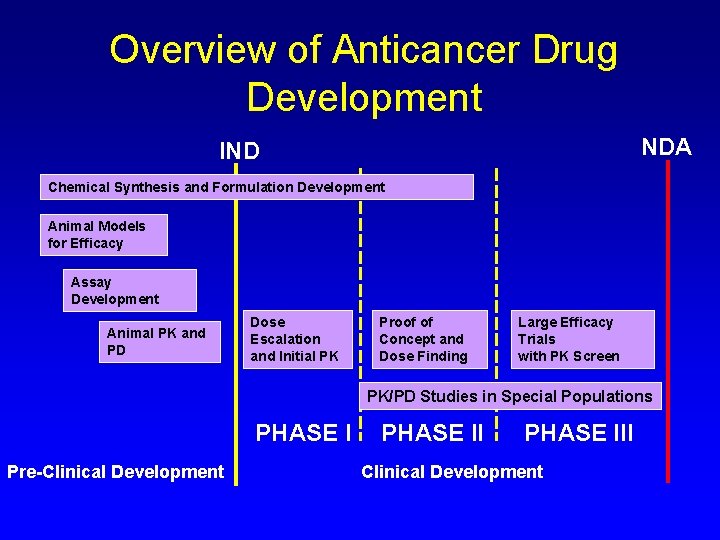



Nonclinical Drug Development Chris H Takimoto Md Ph




Integrated Lab Performance And Biopharma Solutions Eurofins Scientific
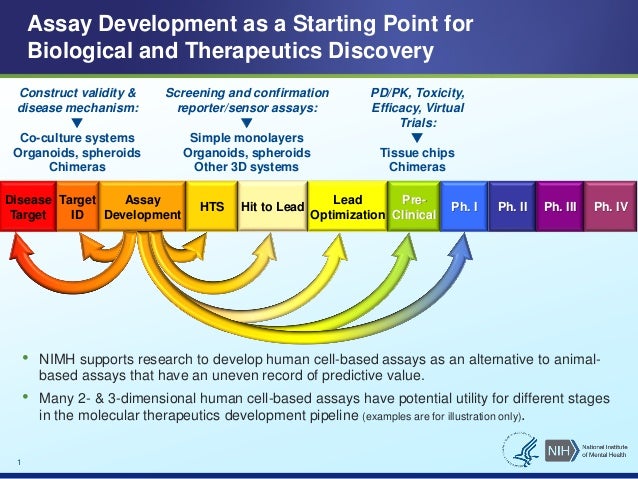



Nimh I Psc Assays For The Drug Pipeline Panchision




A Case For One Assay Analysis Of Pk And Immunogenicity In Biosimilar Research Contract Pharma




Assay Development In Drug Discovery Uab Assay Development In Drug Discovery Southern Research Drug Pdf Document




Application Note A Simoa Pharmacokinetic Bridging Assay Drug Target Review




Pk Pd Modeling Of Protein Drugs Implications In Assay Development Bioanalysis
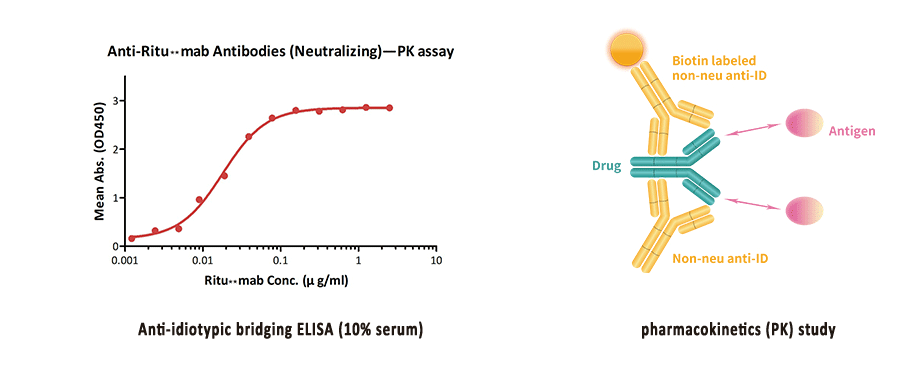



Innovation Lab Capabilities Acrobiosystems




Enzyme Fragment Complementation Technology




Tutorial On Monoclonal Antibody Pharmacokinetics And Its Considerations In Early Development Abstract Europe Pmc




Pharmacokinetic Pk Assays Pk Pd Analysis Dmpk Assay




E Book Potency Assay Guide 2nd Edition Absorption Systems
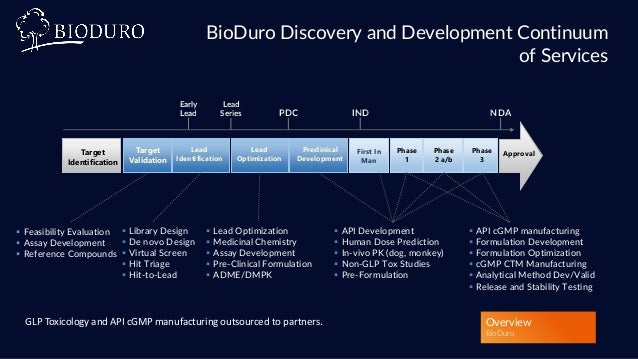



Bioduro




Using Scientist For Contract Pharmaceutical Research Outsourcing Scientist Com




In Vitro And In Vivo Assessment Of Adme And Pk Properties During Lead Selection And Lead Optimization Guidelines Benchmarks And Rules Of Thumb Assay Guidance Manual Ncbi Bookshelf
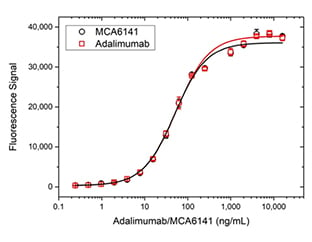



Biosimilar Antibodies For Research Use Bio Rad




Specialty Lab Biomarker Assays Precision For Medicine



Pdf The Assay Design Used For Measurement Of Therapeutic Antibody Concentrations Can Affect Pharmacokinetic Parameters Case Studies




Receptor Occupancy Ro Assays Cellcarta




Pharmacokinetics In Drug Discovery Ruiz Garcia 08 Journal Of Pharmaceutical Sciences Wiley Online Library




Drug Discovery And Development Flashcards Quizlet



X Chem Our Science Lead Optimization



Assay Development In Vitro And In Vivo Taiwantrade Com



Method Development Of A Novel Pk Assay For Antibody Conjugated Drug Measurement Of Adcs Using Peptide Linker Drug Analyte Springerlink




Ligand Binding Assay An Overview Sciencedirect Topics



Method Development Of A Novel Pk Assay For Antibody Conjugated Drug Measurement Of Adcs Using Peptide Linker Drug Analyte Springerlink




Drug Discovery Basic Research Drug Development



Www Uab Edu Medicine Adda Images Padmalayamassaydevelopment Pdf



Developing A Quantitative Surrogate Peptide Assay Peptide Mapping Through Mrm Optimization For Measuring Dulaglutide In A Rat Pk Study Waters




Pk Pd Modeling Of Protein Drugs Implications In Assay Development Bioanalysis




Pharmacokinetic Assay An Overview Sciencedirect Topics
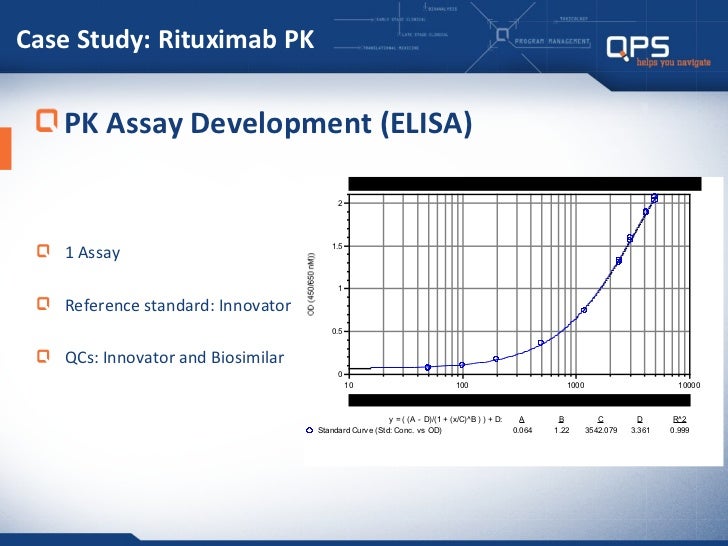



Qps Biosimilar Bioanalytical Approaches




Pdf Ligand Binding Assays In The 21st Century Laboratory Recommendations For Characterization And Supply Of Critical Reagents
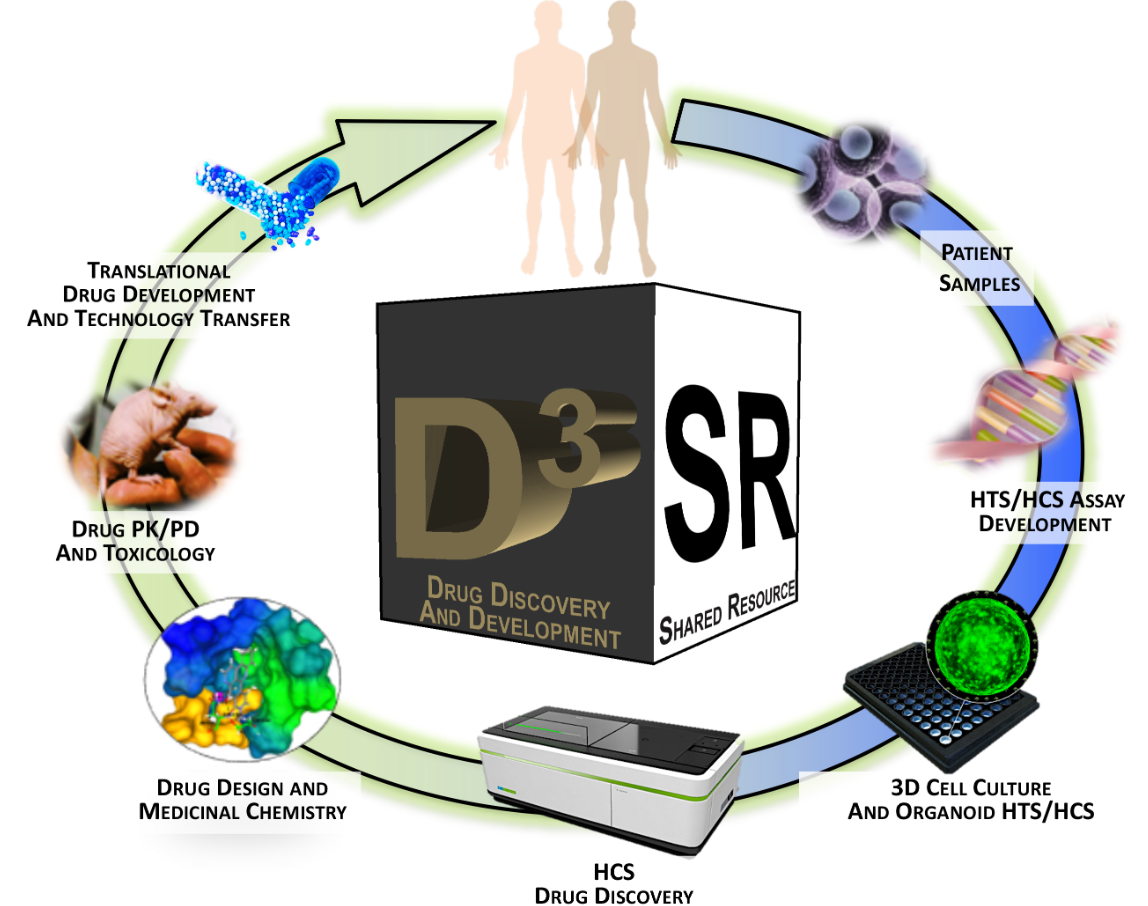



Ilab Organizer Drug Discovery And Development Shared Resource



Development Of A Mass Spectrometry Based Pharmacokinetic Pk Assay For The Measurement Of Rlip76 Protein And Proteoliposome In Human Serum Cellcarta




Receptor Occupancy Ro Assays Cellcarta




Evaluation Of Multiple Immunoassay Technology Platforms To Select The Anti Drug Antibody Assay Exhibiting The Most Appropriate Drug And Target Tolerance




Tutorial On Monoclonal Antibody Pharmacokinetics And Its Considerations In Early Development Ovacik 18 Clinical And Translational Science Wiley Online Library
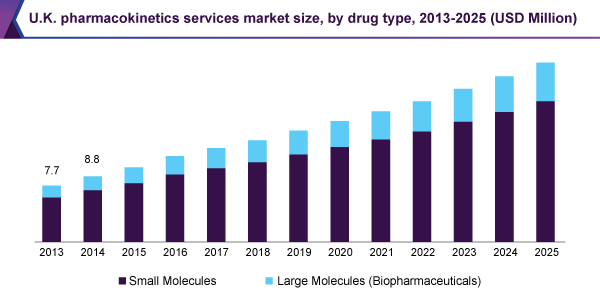



Pharmacokinetics Services Market Size Industry Report 18 25




Managing The Impact Of Immunogenicity In An Era Of Immunotherapy From Bench To Bedside Journal Of Pharmaceutical Sciences
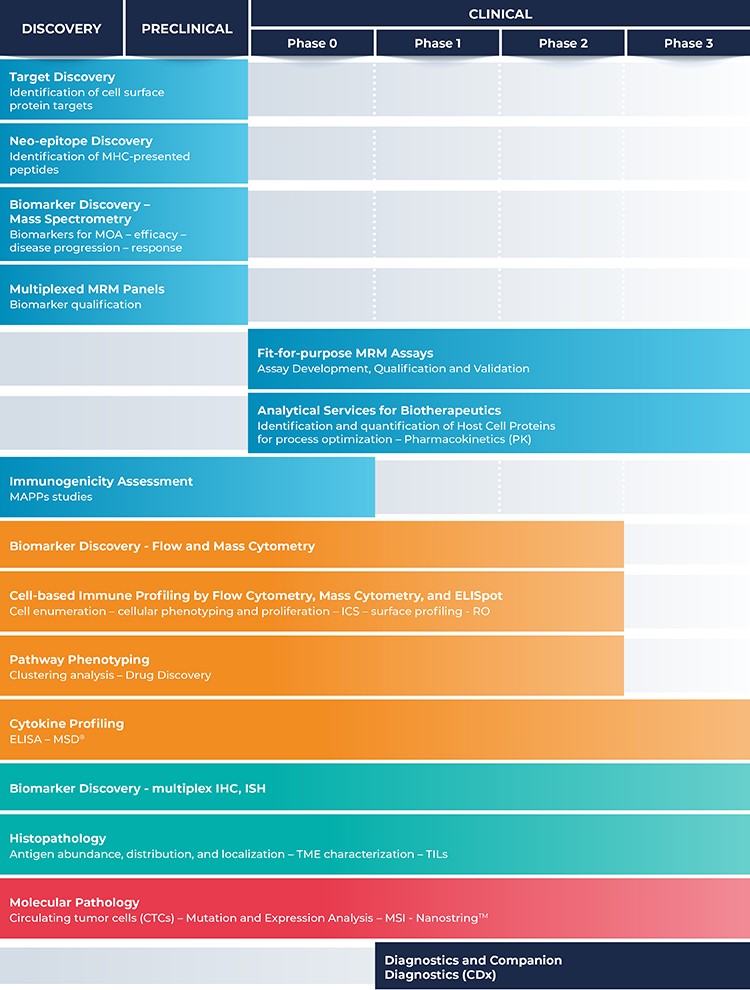



Discovery Phase Cellcarta




Pharmacokinetic Assay An Overview Sciencedirect Topics



Q Tbn And9gcsw7npyr6dolfhphe1nrpexwsvxavlae Axibq4yiix14n Hnpj Usqp Cau



Abclon



Research Utexas Edu Wp Content Uploads Sites 6 16 08 Drug Discovery Development Process 2 Pdf




Smc Immunogenicity Testing Assay Life Science Research Merck



Pharmacokinetic Pk Assays Pk Pd Analysis Dmpk Assay




Ligand Binding Assay An Overview Sciencedirect Topics



2
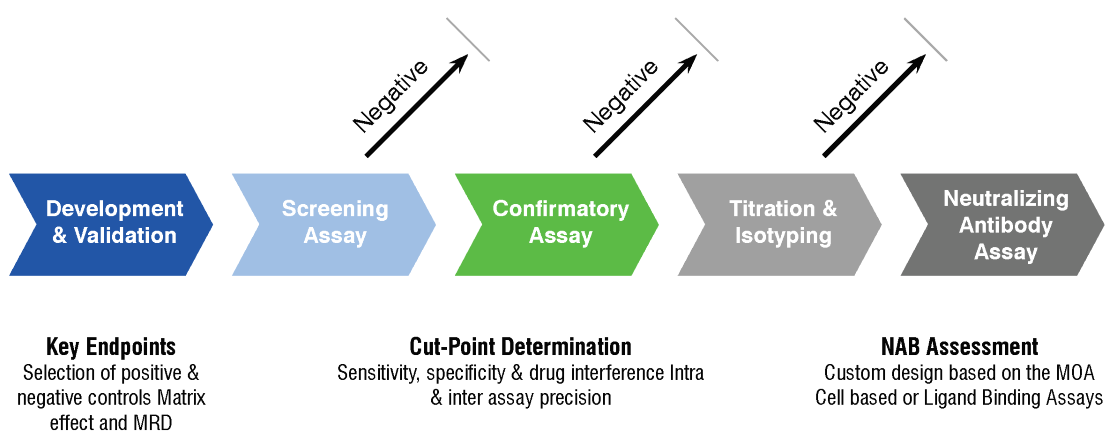



Immunogenicity Testing Charles River



Full Article Bioanalytical Strategy Used In Development Of Pharmacokinetic Pk Methods That Support Biosimilar Programs



Www E B F Eu Wp Content Uploads 18 06 Fw1606 03 Marianne Scheel Fjording Ebf Pdf



1




Using Scientist For Contract Pharmaceutical Research Outsourcing Scientist Com
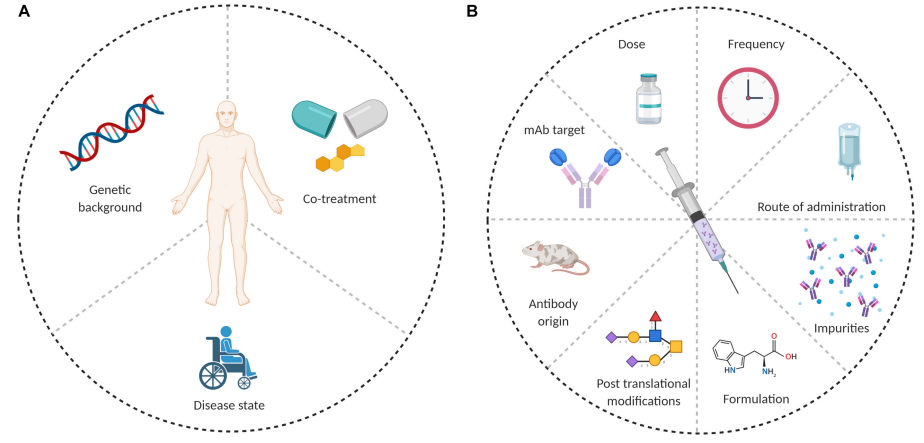



Anti Drug Antibodies Development Creative Biolabs




0olvnr Yzrlsnm




Pharmacokinetic Pk Assays Pk Pd Analysis Dmpk Assay




Receptor Occupancy Assessment By Flow Cytometry As A Pharmacodynamic Biomarker In Biopharmaceutical Development Liang 16 Cytometry Part B Clinical Cytometry Wiley Online Library
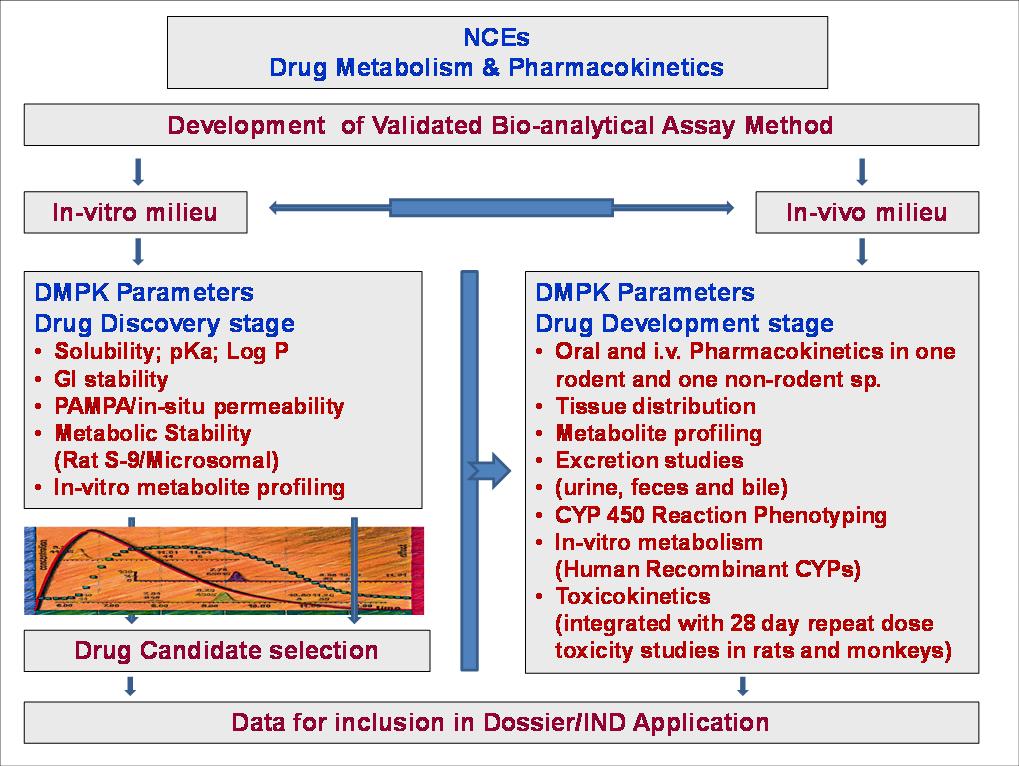



Csir Cdri Home




Chemical Assay Of Drugs And Drug Metabolites Sanford




The Alignment Of Major Pk Pd Related Studies With The Decision Points Download Scientific Diagram



Resources Perkinelmer Com Lab Solutions Resources Docs App Alphalisa Pharmacokinetic Tnfa Pdf



Resources Perkinelmer Com Lab Solutions Resources Docs App Alphalisa Pharmacokinetic Tnfa Pdf




Pk Assay Development For Biologics Using Elisa Msd Immunoassay Platforms



Establish Drug Pk Analysis Method Based On Competitive Elisa




Phases Of Drug Development Process Drug Discovery Process Northeast Biolab




Nab Tab Ada Assays Precision For Medicine



Pk Assay Analysis Services Pharmacokinetics Pk Study Pk Testing Northeast Biolab
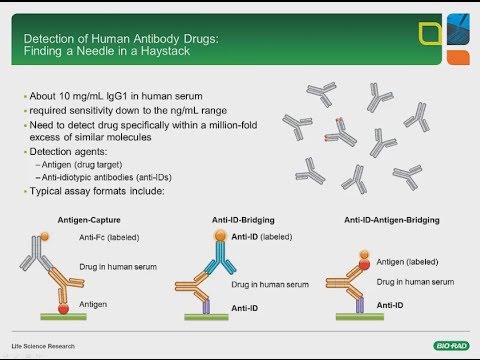



Developing Recombinant Anti Idiotypic Antibodies For Pk Pd And Immunogenicity Assays Youtube




Scielo Brazil Non Clinical Studies In The Process Of New Drug Development Part Ii Good Laboratory Practice Metabolism Pharmacokinetics Safety And Dose Translation To Clinical Studies Non Clinical Studies In The



Pk And Ada Assay Development Services




Figure 3 From Development Of Pharmacokinetic Pk Assays For Detecting Biosimilars Targeting Tnfa Using Alphalisa Semantic Scholar




Ada Immunogenicity Assay Development Kcas Bioanalytical Services
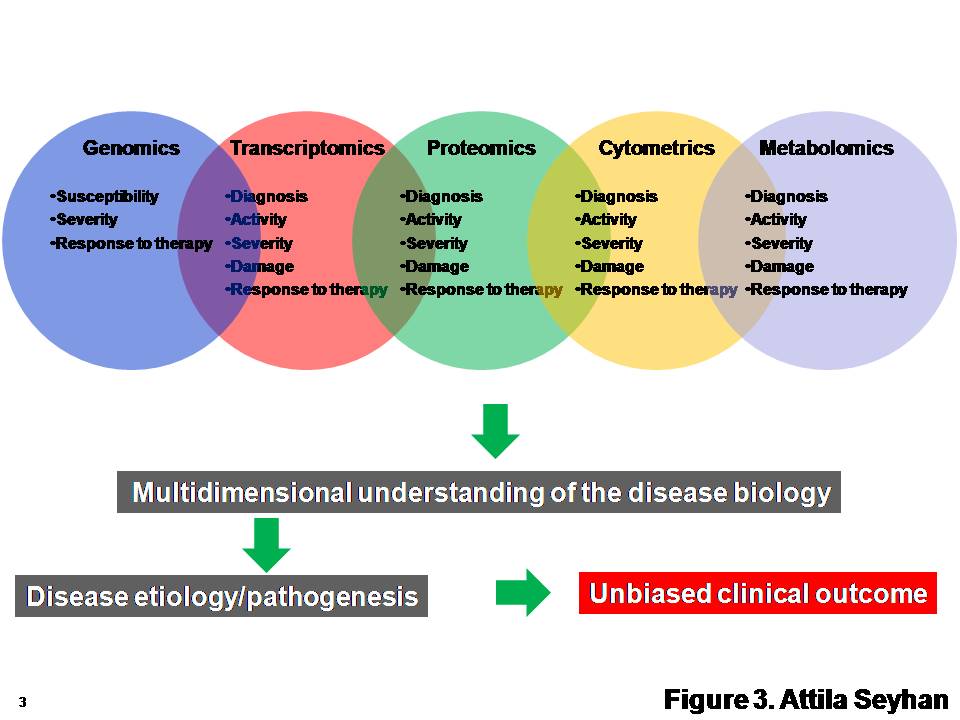



Biomarkers In Drug Discovery And Development




Parallelism Summary Download Table




Pk Pd Modeling Of Protein Drugs Implications In Assay Development Bioanalysis



0 件のコメント:
コメントを投稿